Abstract
Background: Duvelisib (DUV) is a first-in class, oral dual inhibitor of PI3K-δ,-γ being developed for the treatment of advanced B- and T-cell malignancies. To date 304 patients (pts) with relapsed/refractory (RR) CLL/SLL have been treated with DUV monotherapy 25 mg BID in 4 studies: Study IPI-145-02 [NCT01476657], a Phase 1 study in advanced hematologic malignancies; DYNAMOTM [NCT01882803], a Phase 2 study in iNHL; DUOTM [NCT02004522], a Phase 3 study in CLL/SLL; and Study IPI-145-12 [NCT02049515], a Phase 3 crossover study from DUO.
Here we present pooled efficacy and safety analyses from these 4 studies in RR CLL/SLL, examining the baseline characteristics, incidence and timing of AEs, and response in the subset of pts who received DUV monotherapy for >2 years in order to characterize the factors that contribute to long-term treatment with DUV.
Methods: In this analysis we examined pts treated with DUV for >2 years (n=45) referred to as long-term (LT) pts. Efficacy and safety data from 4 studies of DUV monotherapy in pts with RR CLL/SLL treated at 25 mg BID were pooled. Response was based on investigator assessment per IWCLL/IWG criteria. Treatment-emergence (TE) was defined as those adverse events (AEs) that occurred from first dose to 30 days post last dose of DUV. All AEs were coded using MedDRA version 16.1; severity was assessed by investigators according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.03.
Results: Baseline characteristics of LT pts were similar to pts who discontinued <2 years. The median age for LT pts was 68 years (range: 45-90 years); 58% were male and 20% had del(17p) and/or TP53 mutation. LT pts had a median of 2 prior therapies, with 44% having received ≥3 prior therapies. Most pts (76%) had prior chemoimmunotherapy (alkylator based [73%]; purine based [38%]).
The median exposure for LT pts was 31 months, with 20 pts on DUV >3 years and 3 pts on DUV >4 years. The majority of LT pts (60%) remain on treatment as of June 2018.
The median PFS in LT pts was 37 months, with an investigator-assessed ORR of 89%; best responses included 16% CR/CRi, 73% PR, and 11% SD.
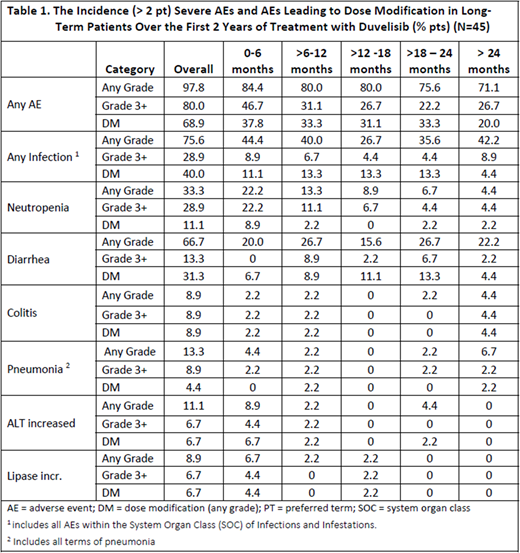
Among LT pts, 98% had an AE, 80% had a ≥ Gr 3 AE, 46% an SAE, and 9% an AE leading to discontinuation (after 2 years on DUV); 69% of pts had at least 1 dose modification due to an AE (69% hold, 40% reduction).
Table 1 shows the incidence of AEs in LT pts by 6-month treatment intervals. Overall, ≥ Gr 3 AEs occurred less frequently over time but still occurred in 27% pts after 2 years. The rate of colitis was consistent over the 2 years of treatment (≤ 2.2% per 6-month interval). No ≥ Gr 3 AEs of diarrhea were observed within the first 6 months of treatment, while the overall rate of all grade diarrhea was consistent over time (16-27% per 6-month interval); dose modification for the management of diarrhea increased over time allowing pts to remain on therapy. Neutropenia, ALT increase, and lipase increase were more common in the first 6 months; most ≥ Gr 3 AEs of neutropenia did not require any dose modification. Compared to all pts with RR CLL/SLL treated with DUV (n=442), the incidences and types of AEs in LT pts were generally similar.
Four (9%) LT pts discontinued DUV due to an AE, and included: colitis (n=2), diarrhea (n=1), Clostridium difficile colitis (n=1), and rectal adenocarcinoma (n=1). Pneumocystis jirovecii pneumonia occurred in 1 LT pt (not on prophylaxis at the time) and was the only opportunistic infection reported for this population. There have been no fatal events in the LT pts.
Conclusions: 45 pts with RR CLL/SLL have been on DUV 25 mg BID for >2 years, with a median of 31 months on treatment. The investigator-assessed ORR for these long-term pts was 89% (16% CR/CRi, 78% PR) and the median PFS was 37 months. A majority (60%) of the long-term pts remain on treatment. Baseline characteristics were similar in these long-term pts compared to pts who discontinued < 2 years. Most of the commonly occurring ≥ Gr 3 AEs decreased over time, with the exception of diarrhea. The majority of ≥ Gr 3 AEs were managed through dose modification (dose interruption and or reduction). These data support DUV monotherapy as a long-term treatment option for pts with RR CLL/SLL with the potential for durable response and good tolerability over time.
Flinn:ArQule: Research Funding; Infinity: Research Funding; Verastem: Research Funding; BeiGene: Research Funding; Curis: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Agios: Research Funding; Forma: Research Funding; Portola: Research Funding; Kite: Research Funding; Forty Seven: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Verastem: Consultancy, Research Funding; Incyte: Research Funding; Janssen: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Research Funding; Pfizer: Research Funding; Trillium: Research Funding; Novartis: Research Funding; Calithera: Research Funding; Constellation: Research Funding. Montillo:Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau. Davids:BMS: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy; Merck: Consultancy; Surface Oncology: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MEI Pharma: Consultancy, Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees. Le:Verastem Oncology: Employment. Lustgarten:Verastem Oncology: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal